


Stay tuned and follow the latest developments and news in Molecular Oncology.

Updated ASCO Guidelines Strongly Recommend Use of the Oncotype DX Breast Recurrence Score® Test in Node-Negative and the Majority of Node-Positive Early-Stage Breast Cancer Patients.
ASCO® has updated its guidelines on biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer.
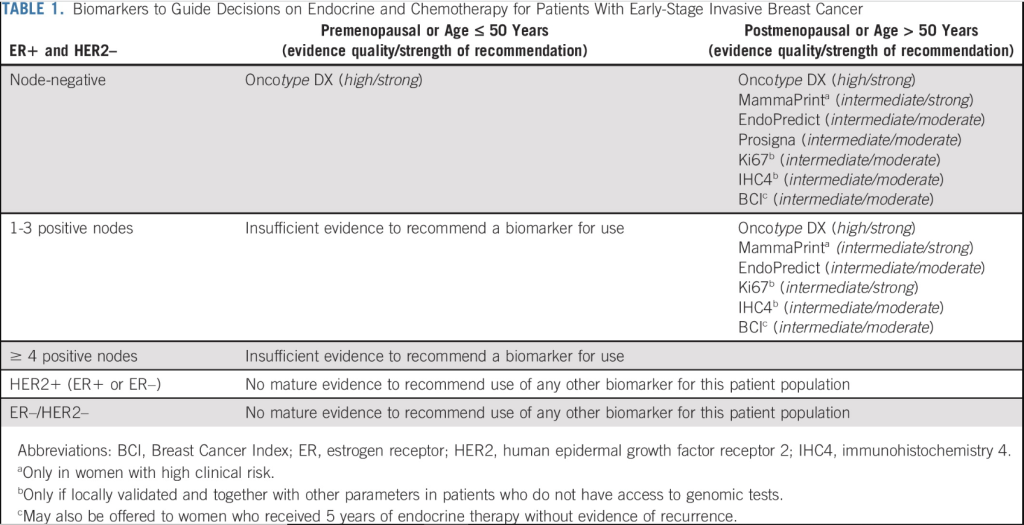
The ASCO guidelines now strongly recommend the use of the Oncotype DX Breast Recurrence Score® test in node-negative and postmenopausal node-positive HR+, HER2-, early-stage breast cancer patients1.
For premenopausal node-negative patients the Oncotype DX® test is the only recommended assay. The recommendation is broad and irrespective of any clinical risk classification.
In addition, the Oncotype DX® test is the most strongly recommended with the highest evidence quality of all genomic assays included in the guidelines.
As published:
1. The ONLY test recommended for use in Premenopausal patients with N0 is Oncotype DX®!!!
2. Also, for the post-menopausal patients Oncotype DX® is the ONLY ONE with high evidence quality and strong recommendation.
3. Mammaprint has a footnote (a) saying that it is ONLY for women with High Clinical Risk which means that it’s is NOT indicated for patients with Grade 1 cancer and smaller than 3cm, Grade 2 and smaller than 2 cm and Grade 3 and smaller than 1 cm.
4. EndoPredict is NOT recommended in premenopausal patients and in post-menopausal patients both in N0 and N1 the strength of recommendation is moderate and NOT strong, due to intermediate quality of evidence and lack of any prospective data.
5. Prosigna is NOT indicated for Node positive patients so it’s indication is suitable ONLY for Node negative Postmenopausal patients.
Find below the paper:
Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update