


Gene tests for neurological disorders
Cerebrum DX® Gene tests for neurological disorders are a series of comprehensive and clinically verified, highly sensitive and specific multi-gene tests that analyze genes associated with hereditary neurological and developmental disorders:
| A sample from the examinee is sent to the laboratory |
| Obtain a family tree - Prenatal counseling |
| Processing of the sample in the laboratory |
| Results in 3-5 weeks - Genetic update |
CerebrumDX ® genetic tests offer the doctor maximum differential diagnosis, with high rates of sensitivity and specificity, saving valuable time and cost per patient case.
Diagnosis: Confirms the clinical diagnosis in a reliable and fast way and reduces the need for more invasive procedures (eg muscle biopsy, lumbar puncture, EMG). It is a useful tool for applying precision medicine to hereditary neurological disorders, minimizing dilemmas regarding the management of differential diagnosis, especially in disorders where the symptoms are milder, such as Myotonic Dystrophy Type 2 or the diagnosis is more demanding, as in Spinal Muscular Atrophy and Autosomal dominant Cerebellar Ataxia.
Prevention: By knowing exactly the gene mutations that are responsible for a patient’s disorder, we are able to predict its course, taking into account all the factors that concern the specific patient. We can also identify relatives who carry the same mutation and are at increased risk of developing the same disorder and be properly managed as appropriate. The contribution of the genetic test to disorders such as Myopathies is very important, where it helps in the prognosis of the patient but also in the monitoring and management of the disease within the family, indicating the need for prenatal control in each case. It also helps with prenatal/pre-implantation screening, upon request of the examinee.
Management: The proper management of the patient and his/her family is based on the reliability of the diagnosis and prognosis of the disease, so that the physician is oriented towards the most appropriate treatment and adjustment. The genetic test offers an immediate answer to the physician as to the most appropriate therapeutic approach per patient case. A typical example is Duchenne Muscular Dystrophy, where diagnosis and management are based on genetic testing of the patient to detect and repair the dystrophin gene mutation.
| PHENOTYPE | GENES | TYPE OF TREATMENT | PHARMACEUTICAL SUBSTANCE |
|---|---|---|---|
| Muscular Dystrophy Duchenne/ Becker (DMD) | Dystrophin | Targeted Therapy | Eteplirsen, Golodirsen, _ Ataluren, Casimersen |
| Spinal muscular atrophy | SMN1/SMN2 | Targeted Therapy | Nusinersen, Onasemnogene abeparvovec, Risdiplam |
| Pigmentosa Retinitis | RPE65 | Targeted Therapy | Voretigene, Neparvovec |
| Leber congenital amaurosis | RPE65 | Targeted Therapy | Voretigene, Neparvovec |
| Amyotrophic Lateral Sclerosis (ALS) | SOD1 | Targeted Therapy | Tofersen |
| Familial Amyloid Polyneuropathy FAP | TTR mRNA | Targeted Therapy | Inotersen |
Cerebrum® gene testing is performed on a blood sample (2 vials of blood with EDTA)
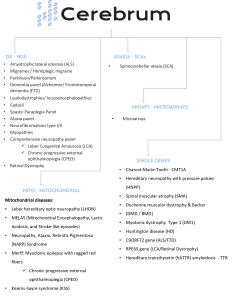
Cerebrum® DX enables the analysis of over 5,200 genes associated with various neurological diseases. It is a comprehensive test for the diagnosis, prognosis and management of neurological diseases, such as Familial Spastic Paraplegia, Parkinson’s, migraines and others.
The iGenome® gene test analyzes the entire genome and aims to find variants in genes associated with inherited diseases, helping the physician in the diagnosis of diseases with unclear phenotypes, but also in prenatal screening.
MyWES® analysis analyses all coding regions of the human genome (over 20,000 genes – Whole Exome Sequencing) and is recommended for the diagnosis and management of cases where there has been no finding in gene panels, as well as in cases the diagnosis is particularly demanding as in the case of ALS – Amyotrophic Lateral Sclerosis.
| ASSAY | GENES | RESULTS TIME |
|---|---|---|
| Cerebrum® DX | >5,000 Genes | 25 BUSINESS DAYS |
| iGenome® | Whole Genome Sequencing | 35 WORKING DAYS |
| MyWes® | Whole Exome Sequencing | 25 BUSINESS DAYS |
To download the Family History table please click here.
The Cerebrum® Ataxia analysis was created for the diagnosis and management of inherited ataxias.
Autosomal dominant cerebellar ataxias: They are caused by triplet repeat expansion of CAG nucleotides and SCA3, SCA6, SCA1, SCA2, SCA7 and SCA 8 are in descending order. In the Greek population, the subtypes SCA7 and SCA1 are most commonly observed. Also, the presence of SCA8 expansions has been observed in the Greek population. This subtype is due to an expansion of a combination of triplet CTA/CTG nucleotides located in a non-coding region of the gene.
On a negative result, the Cerebrum DX® assay is recommended.
| ASSAY | GENES & TYPES OF DISORDERS | RESULTS TIME |
|---|---|---|
| Cerebrum® Ataxia | FXN, SCA1, SCA2,SCA3,SCA6, SCA7 & SCA8 | 10 BUSINESS DAYS |
To download the Family History table please click here.
The Cerebrum® Arrays analysis is based on the Microarrays, method, where chromosomal abnormalities are analyzed and we can identify and diagnose developmental disorders and intervene accurately in their management.
| ASSAY | ANALYSIS OF LARGE GENE REARRANGEMENTS | RESULTS TIME |
|---|---|---|
| Cerebrum® Arrays | CytoScan 750K Suite 750K markers for gene rearrangement analysis (including 200K SNPs and 550K non-polymorphic markers) | 15 BUSINESS DAYS |
To download the Family History table please click here.
Cerebrum® Mito analysis is based on next generation sequencing (NGS) technology enabling the complete sequencing of 37 mitochondrial DNA genes. It is a comprehensive test for the diagnosis, prognosis and management of mitochondrial diseases such as Mitochondrial Encephalomyopathy (MELAS), Myoclonic Epilepsy with Serrated Red Fibers Syndrome (MERFF), Kearns-Sayre Syndrome (KSS) etc.
| ASSAY | MITOCHONDRIAL DNA ANALYSIS | RESULTS TIME |
|---|---|---|
| Cerebrum® Mito | Mitochondrial Diseases such as: Leber/LHON, MERFF, Leigh, MELAS, MELAS, NARP, CPEO - | 25 BUSINESS DAYS |
To download the Family History table please click here.