


A pioneering test for the early detection and early diagnosis of pancreatic cancer
A pioneering test for the early detection and early diagnosis of pancreatic cancer
Avantect® is a liquid biopsy gene test that detects genetic and epigenetic mutations in free circulating tumor DNA (cfDNA) associated with pancreatic cancer. Using this method we can detect pancreatic cancer faster than other methods, managing to detect the cancer at an early stage.
Widespread screening for pancreatic cancer is performed in specialized centers and is usually not feasible due to the lack of effective and affordable diagnostic tests.
Pancreatic cancer becomes more common with increasing age, with over 90% of cases diagnosed in people over the age of 50
In about 90% of cases, pancreatic cancer is sporadic, with the remaining 10% having a genetic origin.
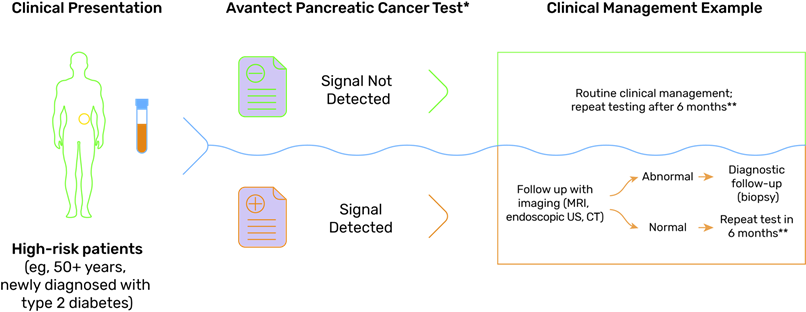
Prioritizing a significant high-risk population
<While diabetes has long been recognized as a risk factor for pancreatic cancer, there is new evidence of a critical relationship between pancreatic cancer and newly diagnosed (3 years) increases the risk and may play a causative role, new-onset diabetes may actually be a consequence of pancreatic cancer. (1% of patients with new-onset type 2 diabetes over the age of 50 will be diagnosed with pancreatic cancer in the next 3 years).
Screening people with a family history of pancreatic cancer
Based on international guidelines, screening for pancreatic cancer is recommended in:
Optimized performance in high-risk patients
The Avantect® test has been validated in patients at high risk of developing pancreatic cancer, including those aged 50 years or older who have been recently < 3 years) diagnosed with type 2 diabetes and those with a family history.
The Avantect® test has a sensitivity of 66.7% for the detection of early stage I & II pancreatic cancer and a specificity of 96.9%.

Who is the Avantect Pancreatic Cancer Test for?
The Avantect® Pancreatic Cancer Test is intended for patients at high risk for pancreatic cancer. For example, it may be recommended for patients ≥50 years of age with newly diagnosed (within 36 months) type 2 diabetes as part of a disease management effort to detect pancreatic cancer.
How long does it take to get my results?
15 business days
What type of sample is needed for the test?
The Avantect® test for pancreatic cancer requires 10 ml of blood for each of the two Cell-Free DNA BCT® Streck tubes provided by Genekor in the test kit.
Is the test covered by public/private insurance?
For information about the cost coverage of the test you should contact your insurance company or our company.
How can I send my sample?
Genekor is responsible for all necessary procedures for the receipt and return of your sample. To arrange sample collection and return, please contact us.
How can I make the payment?
Payment can be made via bank transfer or credit/debit card.
How will I get my results?
Your results will be shared with your doctor via a secure network and to you via e-mail.
Our Customer Service Team is committed to answer your questions with regards to the services offered by Genekor. If you would like to order any of the tests that Genekor performs please contact us directly.
*To complete the test, you are required to complete and send the Consent form that you will find on the link below.
For more scientific information please contact: scientific.support@genekor.com
*Download the promotional brochure here.