


Multigene Liquid Biopsy Test for postmenopausal women with recurrent or metastatic breast cancer ER+, HER2-
The Com.pl.i.t DX® Liquid Breast test is specifically designed for postmenopausal women with ER+ and HER2- breast cancer. This test is a powerful tool for assessing breast cancer status and can be used to decide whether or not a patient should undergo specific targeted treatments.
In more detail, the test is highly recommended for targeted treatment decisions for:
10 Gene Alterations
| Hotspot genes (appr. 152 hotspots:) | AKT1 | EGFR | ERBB2 | ERBB3 | ESR1 | FBXW7 | KRAS | PIK3CA | SF3B1 | TPS3 |
Copy Number Variation- CNV
| Copy number genes (CNVs): | CCND1 | ERBB2 | FGFR1 |
| Full length genes | TP53 |
In the gene panel above, we detect mutations in the ESR1 gene (incidence 48% as a mechanism of resistance to hormone therapy, based on the Emerald study).
We also detect PIK3CA mutations with the approved treatment Alpelisib, as well as other genes for which there are experimental or off-label treatments.
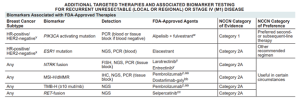
BIOMARKERS ASSOCIATED WITH FDA APPROVED THERAPIES

What types of cancer does it cover?
Covers recurrent or metastatic breast cancer ER+/HER2-
What is the sample type required for the Com.Pl.i.t DX® Liquid Breast analysis?
Blood in a special STRECK vial supplied by Genekor. Blood in special collection vial that you are going to take from Genekor. (Cell-Free DNA BCT®(10ml) και Cell-Free RNA BCT®(10ml)).
How do I get the special vial?
Genekor is responsible for providing and shipping the special vial for the Com.Pl.i.t DX® Liquid Breast test. Please contact us.
Are there any special instructions for collecting a blood sample?
Yes, click here for more information on the specific instructions for collecting a blood sample.
How do I send my sample and receive my results?
Genekor is responsible for all necessary procedures for the receipt and return of your sample.
Your results will be shared with your doctor via a secure network and to you via e-mail with a secure unique code provided by customer service.
In how many days will I receive my results?
Results will be available in 10 working days from the day after your sample is received.
Is the test covered by any public / private insurance?
For information about the cost coverage of the test you should contact your personal insurance or our company.
How can I make the payment for the test?
The Customer Service Department will provide you with a unique e-banking payment code, or payment can be made by card or bank transfer.
Why do I have to sign the consent form?
GeneKor Medical SA is certified according to ISO 9001:2015 (Cert. No. 041150049) and according to ELOT ISO/IEC 27001:2013 (Cert. No. 048190009) by TUV NORD HELLAS, which require the written consent of each patient for the use of their genetic material for testing.
It is also necessary in accordance with data protection regulations.
Our Customer Service Team is committed to answer your questions with regards to the services offered by Genekor. If you would like to order any of the tests that Genekor performs please contact us directly.
*To complete the test, you are required to complete and send the Consent form that you will find on the link below.
*For more information on scientific content please contact: scientific.support@genekor.com
*Download the promotional brochure here.
*Order in the United Arab Emirates here.